21 August 2020
Webinar on Detection and Identification of Substandard and Falsified Medicines in Nairobi, Kenya
Event
21 August 2020

On 20 and 21 August 2020, MEDISAFE organized a webinar in Nairobi, remotely conducted from Paris at Expertise France HQ. This webinar aimed at strengthening the detection and identification of Substandard and Falsified Medicines (SFM), products and SF Medical Materials and Consumables (SFMMC) in the specific context of COVID-19 to improve stakeholders’ capacities. This webinar also contributed to ensure the securing of the supply chain in accordance with international/national regulations and facilitate the sharing of best practices.

Participants located in Nairobi attended this virtual training including INTERPOL and EU CBRN. The specific objectives of this training are as followed:
– To detect suspicious documentation, containers, package of SFM and SFMMC products
– To detect frauds, suspicious dysfunctions in the medicine market
– To expose to his various interlocutor’s information on SFM/MMC products and on legal and illegal medicines chain
– To understand the importance of the Quality Control (QC), pharmacovigilance and inspection in the detection of false documentation in the procurement and distribution process
– To define key points to elaborate MEDISAFE action plan
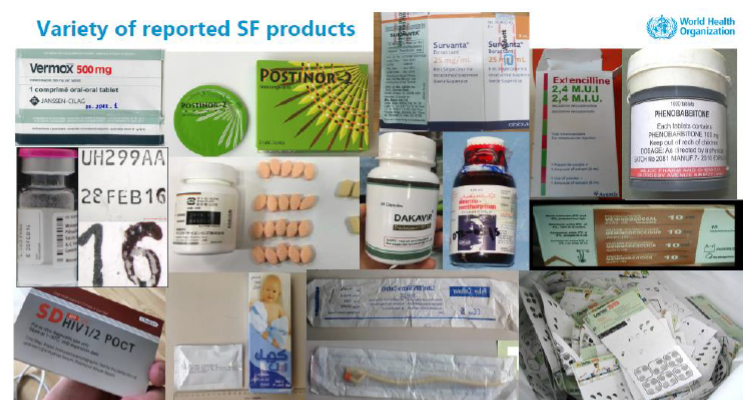
This 2-day training was the opportunity to discuss national regulations in the specific context of the COVID-19 pandemic and ensure the exchange of information at the national, regional and international level.